The concept of a “throwaway society” is one in which consumerism has led to overconsumption and wasteful disposal of products that could – and should – be reused. To an extent, items in the dental office should be reused, but when infection prevention is concerned, there are very good reasons why some items can be reprocessed and why some items are single-use.
Knowing whether products or tools are single-use or reusable is a fairly straightforward issue, but too often, clinicians make the mistake of reprocessing single-use items or, in some cases, reprocessing equipment beyond the manufacturer’s recommendations.
Read the instructions
Some violations may just be chalked up to an honest mistake and simply not knowing any better. But other cases are downright shocking.
“This topic can drive me bonkers,” Karen Daw, “The OSHA Lady”, speaker and consultant, says. “I’ve conducted dental practice consults where disposable prophy cups, suction tips, water syringes, impression trays—and even dental floss—has been found in cold sterile solution. The reason for this? The practice intends to reuse these items.”
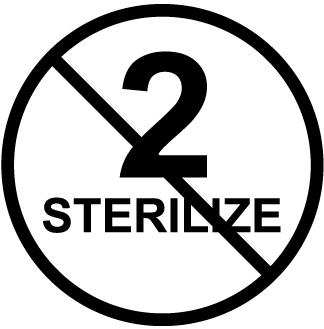
It shouldn’t be difficult to figure out which items can be reprocessed and which are intended only for a one-time use. Single-use items are designated as such and there is a standardized symbol on those items’ packages.
“To determine single-use vs. sterilizable items, the user should reference the MIFU (manufacturers’ instructions for use),” Daw says. “For example, the manufacturer might indicate single-use only by placing a number ‘2’ in a circle with a line through it to indicate that using anything more than once is prohibited. Or, it might plainly state on the box ‘disposable’. Other times, it takes due diligence to figure out whether the items can or should be reprocessed.”
“I think the rationale behind that is that once that mask is used, obviously it’s soiled on the exterior. It has splatters and splashes that could have come out of the patient’s mouth,” Dorst says. “It could have bacteria, viruses, fungus, microorganisms. And once the clinician touches it with their hand, or even their gloved hand, they’ve transferred the germs from the mask to their hand. Then, if they had any aerosol splatters during patient care on their neck area, and they pull that mask down under the chin, contamination is on the inside of the mask. We constantly see, in the media, inaccurate photos showing dental personnel with a mask pulled underneath their chin. So after they do that procedure and then they replace that mask over their nose and their mouth, all of the microorganisms that were on their neck are now transferred to their nose and their mouth. They could acquire an infection through exposure to the mucous membranes from any of those microorganisms.”
While the majority of violations, in this regard, seem to be from reusing single-use items, risks exist on the other end of the spectrum: Not properly processing equipment requiring special attention.
The rules
The manufacturer’s instructions for use provide necessary guidance for which items are reusable and which are not, but they get those rules from an even higher authority.
“The Centers for Disease Control and Prevention (CDC) reference the Food and Drug Administration on disposable devices,” Daw observes. “These are items to be used for one patient during a single procedure and will not be reprocessed for use on any other individual. On this they are very clear: If the device does not have validated reprocessing instructions, it should be considered single-use and disposed of accordingly, regardless of the labeling. If in doubt, contact the manufacturer directly.”
The biggest issue involving whether an item should be reused or not is if the manufacturer indicates that it can be properly sterilized.