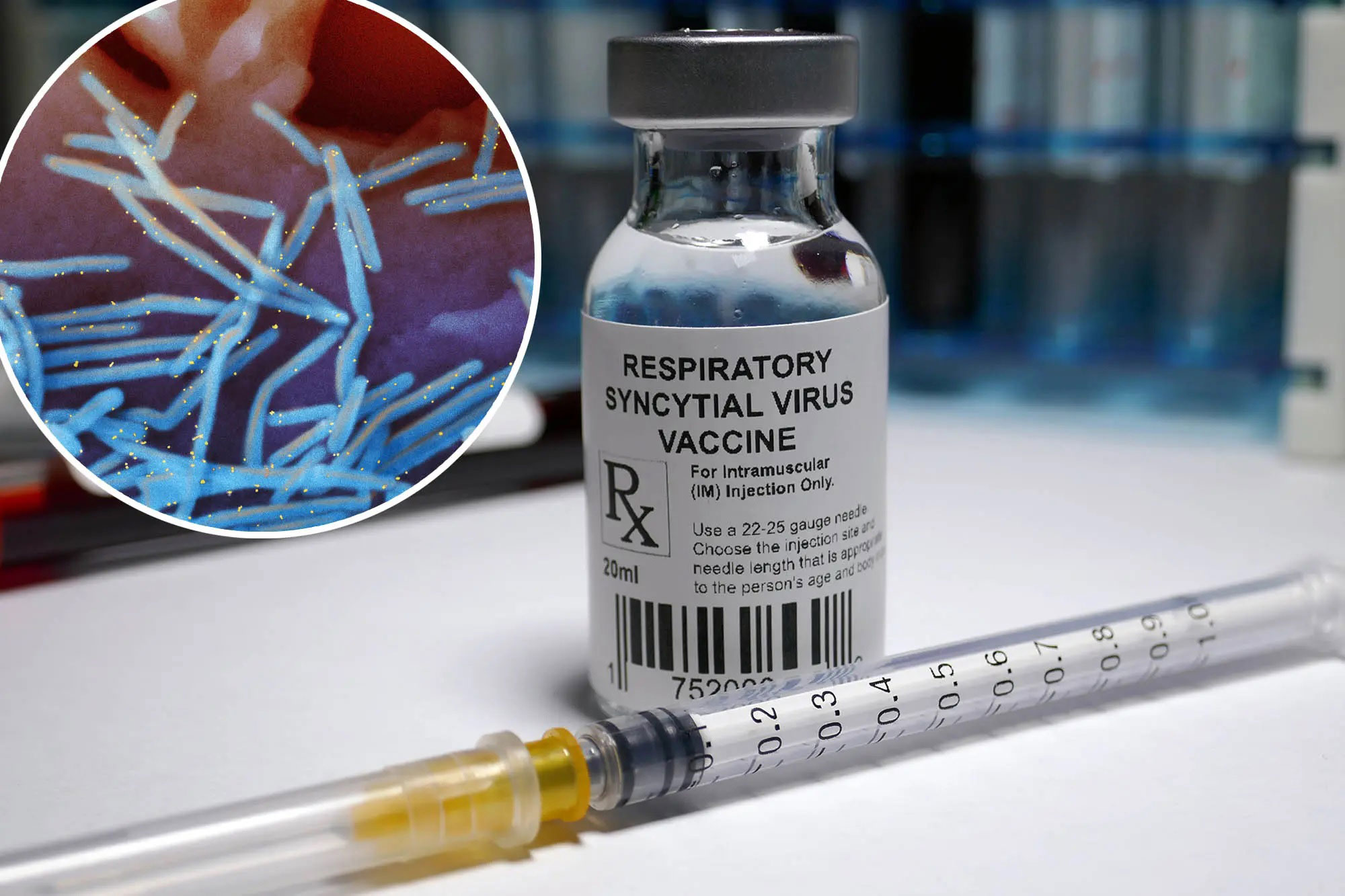
The administration of the first RSV (Respiratory Syncytial Virus) vaccine in infants and pregnant women has recently been introduced as a significant measure for preventing this virus. Here is detailed information on the first RSV vaccines and their administration methods:
Nirsevimab
- Type of Vaccine: Nirsevimab is a monoclonal antibody used to prevent RSV in infants. It provides "passive immunity" by giving the infant antibodies directly.
- Administration: This vaccine is administered as an intramuscular injection in the infant's thigh.
- Efficacy: Studies have shown that Nirsevimab can prevent 90% of hospitalizations due to RSV in infants
- Side Effects: Common side effects include pain, redness, or swelling at the injection site and rashes. Serious side effects are rare.
Maternal RSV Vaccine (Abrysvo)
- Type of Vaccine: This vaccine is administered to pregnant women to transfer protective antibodies to the fetus.
- Administration: The maternal RSV vaccine is given between the 32nd and 36th weeks of pregnancy.
- Efficacy: This vaccine can reduce the risk of hospitalization for RSV by 57% in the first six months of the infant's life
- Side Effects: Side effects include injection site pain, headache, muscle pain, and nausea. Some studies have indicated a slight increase in preterm births among vaccinated mothers.
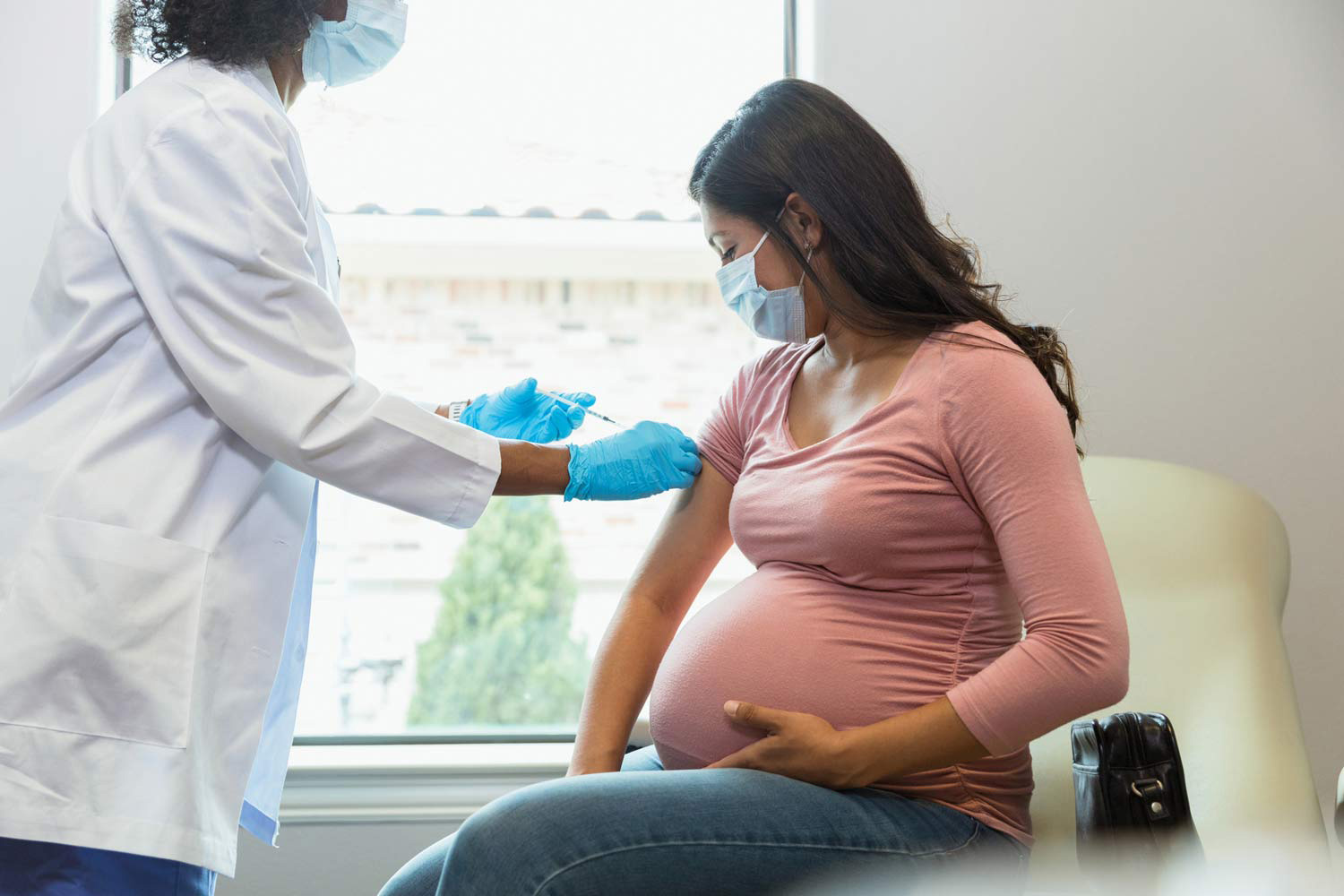
These vaccines and administration methods represent significant steps in preventing RSV-related illnesses in infants and children, potentially reducing the burden of respiratory diseases in this age group.

