-
منوی اصلیبستن
-
Dental
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Dental Finishing & Polishing
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
- Mag
-
Laboratory
-
-
Laboratory Equioment
-
-
-
-
Lab
-
-
-
Medical
-
-
-
-
تجهیزات تشخیص
-
تجهیزات عکس برداری
-
-
-
- Help
-
Corporate
-
- Login/ Register
-
منوی اصلیبستن
-
Dental
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Dental Finishing & Polishing
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
Gloves
-
-
-
- Mag
-
Laboratory
-
-
Laboratory Equioment
-
-
-
-
Lab
-
-
-
Medical
-
-
-
-
تجهیزات تشخیص
-
تجهیزات عکس برداری
-
-
-
- Help
-
Corporate
-
- Login/ Register
- Home
- Medical & Surgical Equipment
- Medical & Surgical Supplies
- Cardiology
- CARDIOVASCULAR SURGERY
- VSD Occluder
- LifeTech Scientific - Cera VSD Occluder
Cera VSD Occluder - LifeTech Scientific Corporation
-
In line with the demand of Dandal site customers for credit purchases, Dandal in cooperation with Saman Bank has provided a new service for dental and medical offices and treatment centers, which customers can use a credit card from the site at any time without any cash support.
- More Info
LifeTech Scientific - Cera VSD Occluder
Out-of-StockFeatures:
Ventricular Septal Defect Occluder
China Made
Nitinol wire frame coated with Titanium Nitride
Pay in installments
Choose your plan:
Current plan: 3Month
Description: 30+3
Down payment: تومان0 (25.00%)
Number of payments: 3
Tax: تومان0
Amount of payment: تومان0
Overpayment: تومان0
Total: تومان0
Current plan: Cash
Description: نقدی
Down payment: تومان0 (0.00%)
Number of payments: 1
Tax: تومان0
Amount of payment: تومان0
Overpayment: تومان0
Total: تومان0
Nitinol wire frame coated with Titanium Nitride (TiN)
Decrease the dissolution of nickel ion efficiently, expected safe long-term biocompatibility.
Promote the growth of endothelial tissue, lessen thrombus complication.
Superior superelastic, effectively reduce atrioventricular block occurrence.
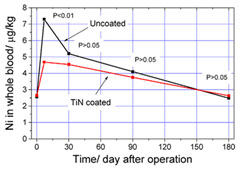
Nickel ion concentration in whole blood
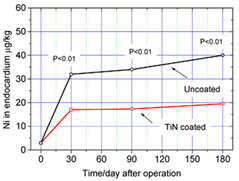
Nickel ion concentration in endocardium
Cera Animal Study: Compared to Nitinol occluder, the Cera occluder has demonstrated that faster and better growth of endothelial cell and lower risk of thrombus formation.
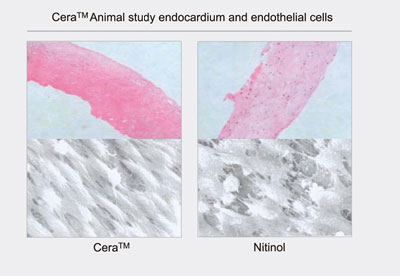
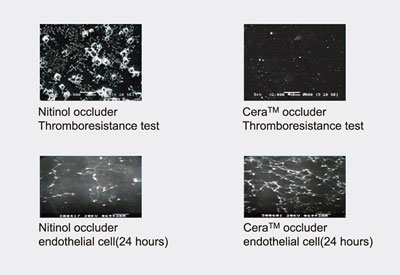
Cera Animal Study: Compared to Nitinol occluder, the Cera occluder has demonstrated that faster and better growth of endothelial cell and lower risk of thrombus formation.
Strategic Membrane Selection
The ASD/PFO covered by a PET membrane that minimizes the chance of clot formation and has a small volume to get into lower profile sheath.
The VSD/PDA occluder covered with a PTFE membrane, which has a denser structure suited for high pressure defect.
Conform anatomical features of the defect, providing optimal design
3 types of peri-membranous VSD and a muscular VSD devices designed for different kind of VSD.
The ASD waist diameter available ranging from 6 to 42 mm, and the PDA occluder ranging from 0406 to 2224.
Cera Clinical study in China
Principal Investigator: Zhang Zhiwei, MD, FACC, Guangdong Cardiac Institute.
Evaluation of efficacy and safety of Cera septal defect occluder for congenital cardiac detect: A multicenter, randomized and controlled clinical trial.
- 11 medical centers,460 cases enrolled (231 Cera , 229 HeartR ).
- Follow up endpoint: 1, 3, 6, 12 months.
Study conclusion
The success rate of immediate complete occlusion with Cera occluder is higher than 97.8%.
The results showed that the incidence of residual shunt were relatively less.
The arrhythmia incidence in Cera group is lower than that in HeartR group which as much as 36%.
Cera PM-VSD clinical study in Brazil
Principal Investigator: Doctor Raul Arrieta and tutored by Doctor Carlos Pedra.
Prospective, multicenter, non randomized study.
Starting on November 2010.
3 Brazilian institutions, 56 patients.
Follow up endpoint: 1, 3, 6, 12 and 24 months.
Initial Result
The percutaneous closure of peri-membranous ventricular septal defect with Cera Device has showed a safe effect with excellent immediate occlusion and low complication.
Leave a comment about this product
Write your review
LifeTech Scientific - Cera VSD Occluder





