Neodent Grand Morse Implant Systems Overview
 |
|
GM - Helix
Hybrid Dual Tapered
All bone type
|
 |
|
|
Diameter 3.5 - 3.75 - 4 - 4.3 - 5
Height 8 - 10 - 11.5 - 13 - 16 - 18
|
|
GM - Drive
Tapered
Bone type III and IV
|
 |
|
|
Diameter 3.5 - 4.3 - 5
Height 8 - 10 - 11.5 - 13 - 16 - 18
|
|
GM - Titamax
Cylindrical
Bone type I and II
|
 |
|
|
Diameter 3.5 - 3.75 - 4 - 5
Height 7 - 8 - 9 - 11 - 13 - 15 - 17
|
|
For more information Click on the above pictures.
|
- Platform switching
- Acqua and NeoPoros Surface
- Possibility of implantation above the bone cause of its adhesion to soft tissue
- Variety of prosthetic components
- Life time warranty
- CoverScrew not include in fixture package
About Neodent company
Founded in 1993, Neodent has over 20-year experience in the design, development, and manufacture of dental implants and related prosthetic components. Its success has been achieved through a philosophy of making tested implant solutions more affordable to a broader population. The proven product concept achieves 99.7% cumulative implant survival rate, supported by more than 200 studies.
Neodent also offers hands-on heavy global education courses specifically focusing on the immediate treatment protocol through a team approach.
In 2012 Straumann, an international leader in the implant sector, purchases a stake in Neodent, beginning an important scientific and commercial partnership. and in 2015 With the anticipation of the total acquisition of 100% of the Company in April, Neodent becomes part of the Straumann Group. The company maintains its independence and headquarters in Curitiba, which also happens to be the headquarters of the Straumann Group for operations in Latin America. The internationalization process is potencialized. The two companies combine their operations in Mexico, to meet the subsidiaries of Central America, and Neodent opens branches in Colombia, to meet Ecuador, Peru and Venezuela, and in Argentina, to meet Chile and Uruguay, as well.. The two companies combine their operations in Mexico, to meet the subsidiaries of Central America, and Neodent opens branches in Colombia, to meet Ecuador, Peru and Venezuela, and in Argentina, to meet Chile and Uruguay, as well.
Grand Morse
The Neodent Grand Morse Implant System is the achievement of more than 20 years of experiences in implant dentistry, and shared experiences with many clinicians worldwide. Continuing with a unique purpose to always deliver high quality treatment options that changes patients’ lives, the Grand Morse Implant System is the Neodent evolution. Anchor within our philosophy of respecting mechanical and biological principles, this makes it THE implant of choice in dental implant therapy.
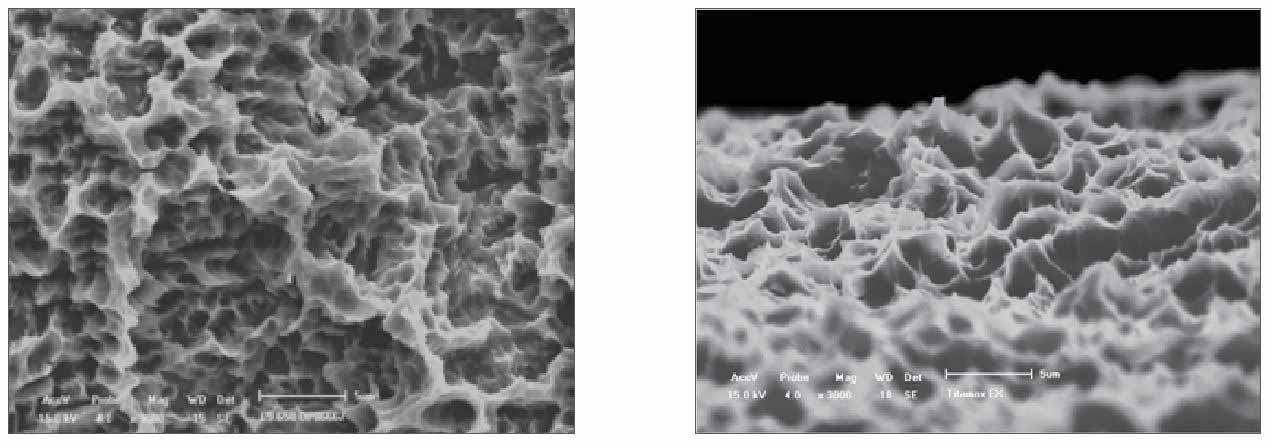
NeoPoros Surface
Constant evolution and safety guarantee.
Based on the abrasive sandblasting concept followed by acid etching, the NeoPoros surface promotes, by using controlled grain oxides, cavities on the implant surface that then are uniformed with the acid etching technique.
The whole process of obtaining this surface is guaranteed due to automated time, speed, pressure and particle size control.
Several scientific studies continue to be performed so that the NeoPoros surface may be always evolving and promoting much more reliability for you.
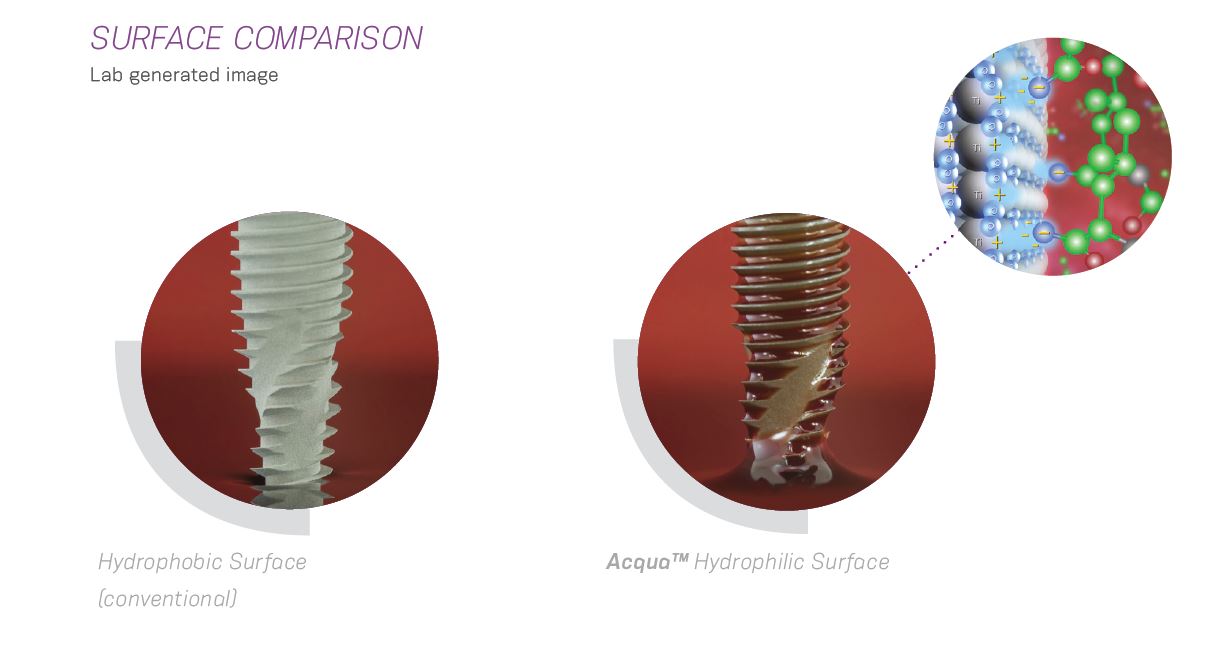
Acqua Surface
Hydrophilic Surface designed for high treatment predictability
The Neodent Acqua hydrophilic surface is the next level of the highly successful S.L.A. type of surface developed to achieve successful outcomes even in challenging situations, such as soft bone or immediate protocols.

The Neodent Grand Morse implants provide a complete range of treatment options to create the optimal tooth replacement outcomes for all indications, from single tooth to fully edentulous:
• Helix Grand Morse is an innovative hybrid implant design maximizing treatment options and efficiency in all bone types.
• Drive Grand Morse implant is a fully tapered implant developed to achieve high primary stability in challenging bone situations such as soft bones and extraction sockets.
• Titamax Grand Morse is a cylindrical implant indicated for bone types I and II and allowing vertical placement flexibility.

1. Helix
Implants Description:
• Full dual tapered implant;
• Hybrid contour with a cylindrical coronal part and conical on the apical area;
• Active apex including a soft Rounded small tip and helicoidal flutes;
• Dynamic progressive thread design: from compressing trapezoidal threads on the coronal area to self-tapping V-shape threads on the apical part;
• Double threaded implant;
• Grand Morse connection.
Indications:
• Indicated for all types of bone density and implant immediate placement post extraction.

2. Drive
Implants Description:
• Tapered implant;
• Square shape threads;
• Double threaded implant;
• Reverse cutting chambers distributed across the implant body;
• Rounded apex with a sharp edge;
• Grand Morse Connection.
Indications:
• Indicated for bone types III and IV and implant immediate placement post-extraction.

3. Titamax
Implants Description:
• Cylindrical implant;
• V-shape threads;
• Double threaded implant;
• Self tapping apex;
• Grand Morse Connection.
Indications:
• Indicated for bone types I and II or grafted areas such as bone block.

Author: Review department of Dandal.ir
First Release Date: 06/Mar/2018
Sources:
(1) Al-Nsour MM, Chan HL, Wang HL. Effect of the platform- switching technique on preservation of peri-implant marginal bone: a systematic review. Int J Oral Maxillofac Implants. 2012 Jan-Feb;27(1):138-45.
(2) Annibali S, Bignozzi I, Cristalli MP, et al. Peri-implant marginal bone level: a systematic review and meta-analysis of studies comparing platform switching versus conventionally restored implants. J Clin Periodontol. 2012 Nov;39(11):1097-113.
(3) Hsu YT, Lin GH, Wang HL. Effects of Platform-Switching on Peri-implant Soft and Hard Tissue Outcomes: A Systematic Review and Meta-analysis. Int J Oral Maxillofac Implants. 2017;32(1):e9-e24.
(4) Lazzara RJ, Porter SS. Platform switching: a new concept in implant dentistry for controlling postrestorative crestal bone levels. Int J Periodontics Restorative Dentistry. 2006 Feb;26(1):9-17.
(5) Rocha S, Wagner W, Wiltfang J, Nicolau P, Moergel M, Messias A, Behrens E, Guerra F. Effect of platform switching on crestal bone levels around implants in the posterior mandible: 3 years results from a multicentre randomized clinical trial. J Clin Periodontol. 2016 Apr;43(4):374-82.
(6) Novellino MM, Sesma N, Zanardi PR, Lagana DC. Resonance frequency analysis of dental implants placed at the posterior maxilla varying the surface treatment only: A randomized clinical trial. Clin Implant Dent Relat Res. 2017;00:1–6.